To study the vast number of possible glycan protein interactions high-throughput tools as known from genomics and proteomics workflows are required. The strength of carbohydrate-protein interactions however is generally far weaker than protein-protein interactions and very sensitive to ligand presentation. Clustering of glycans (e.g. by the dense immobilization on a surface) can increase the affinity significantly allowing the study of thousands of interactions simultaneously using robust array-based assays. We routinely print our collection of synthetic glycans with a particular focus on unique parasite and plant structures, which are underrepresented on other glycan arrays onto glass slides to study relevant host-pathogen interactions with human immune receptors and antibodies and the substrate affinities of glycosyltransferases. We have employed on-chip enzymatic elongations of printed glycans for the rapid generation of glycan arrays with substantial diversity (Serna et al. 2010) and we are currently exploring this methodology for the on-chip synthesis of glycomimetics in collaboration with the group of Anna Bernardi, University of Milan.
By measuring the signal intensity of bound, fluorescently labeled proteins, binding events are usually recorded but to be able to measure enzyme activity on printed glycan ligands we developed multimodal readout techniques that were compatible with fluorescence, mass spectrometry and tritium imaging. Glass slides coated with a transparent and conductive indium-tin oxide thin film were further functionalized with hydrophically bound and NHS activated non-covalent linker. Printing of amino-functionalized glycans resulted in the on-chip formation of neoglycolipids, that can be visualized with excellent sensitivity by MALDI-Tof MS. (Beloqui et al. 2013)
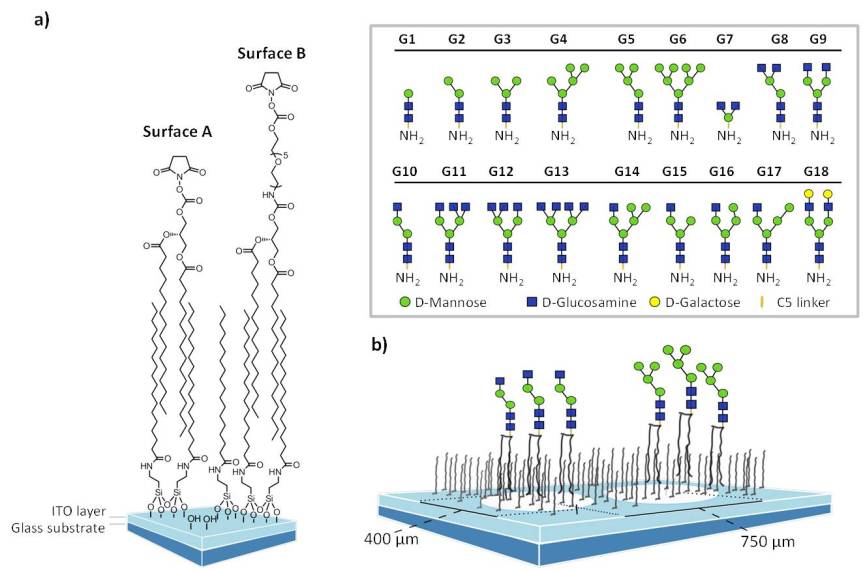
Our current glycan array covers over 130 synthetic glycans structures which are routinely printed on a variety of surface formats.

