Chemo-enzymatic synthesis of N-glycans
The access to pure and well-defined glycan structures is a key requisite for most of our projects and a major effort in our group is devoted to the chemical synthesis of glycans and glycomimetics. To be able to rapidly access large numbers of related of glycan structures we concentrate on the synthesis of core structures that are conserved in a certain class of carbohydrates and employ recombinant enzymes for their rapid and stereo-selective diversification. Most enzymes we employ are not commercially available and were cloned and expressed in-house. To be able to analyze in detail the interaction of pathogen glycans with human immune receptors involved in pathogen recognition and uptake we have focused on the synthesis of parasite glycans that share certain core structures with human glycans but often present highly immunogenic structural elements. As an example we have recently developed a convergent synthesis for a 6 core xylosylated glycans, which were enyzymatically diversified into a library of 33 highly complex N-glycans (Brzezicka et al. 2015)
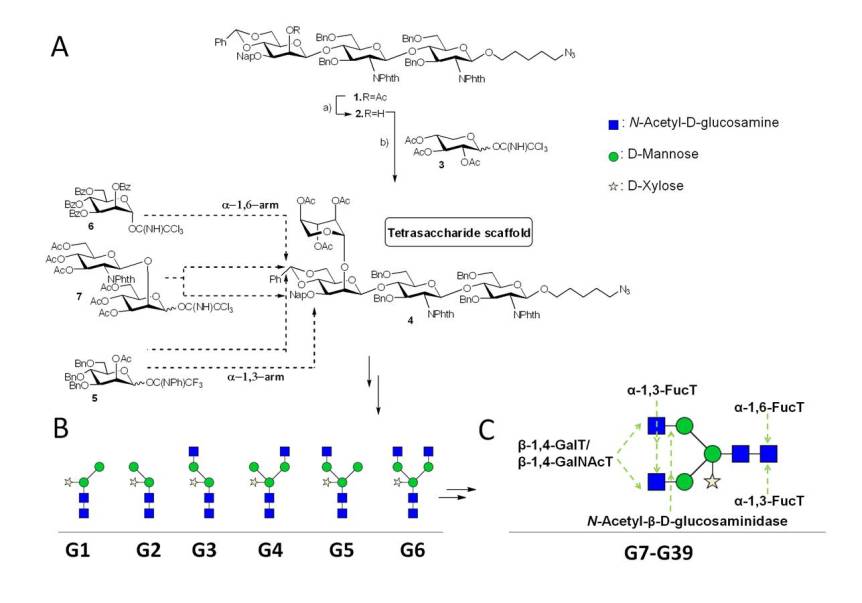
The majority of multi-antennary N-glycan structures found on proteins are incompletely elongated due to a number of factors including the structural accessibility of glycosyltransferases to individual acceptor sites on the glycan, interactions between glycan acceptor with the protein backbone and physiological levels of nucleotide sugar donors and enzymes. By carrying out the reaction on glycan structures acceptors, that are still partially protected with UV-active benzyl groups we can efficiently isolate all or most enzymatic reaction products by preparative HPLC (Echeverria et al. 2015)
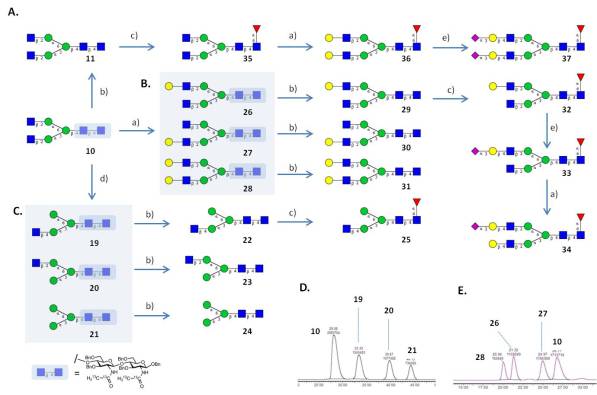
A final traceless hydrogenation provides the reaction products with great ease and purity. We are employing this methodology extensively for the preparation of libraries of stable isotope labeled glycans used as internal standards in clinical glycan biomarker studies.
Solid-phase synthesis of Glycosaminoglycans
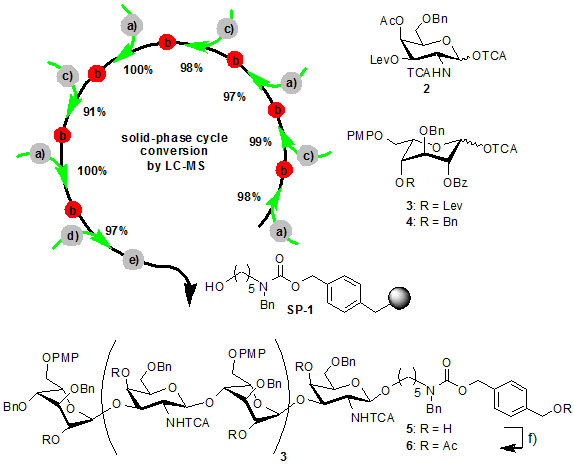
Glycoaminoglycans are linear usually highly sulfated polysaccharides found on the extracellular space of mammalian cells, where they exert import functions in joint lubrication, protein retention and gradient formation and molecular recognition. Heparin is a prominent member of the family of polysaccharides and one of the world´s most important drugs (in volume). It has been speculated that the immense structural variability found in glycosaminoglycans could be a code for selective molecular recognition processes with a wide spectrum of binding proteins but to verify this hypothesis pure structures with defined sulfation pattern, length and monosaccharide sequence are needed. We have chosen a solid-phase strategy to address the synthesis of glycosaminoglycan fragments with well defined composition. Our initial results indicate that the solid-phase assembly of monosaccharide units to glycosaminoglycan oligosaccharides of biologically relevant length (6-8 residues) is feasible with good yields and current work is directed to arrive at the fully deprotected compounds for biological testing. In the future we would like to generate diverse libraries of glycosaminoglycans to study the molecular basis of their interaction with binding proteins.